Abstract
Introduction. Conventional conditioning agents for stem cell transplantation (SCT) and gene therapy are associated with off-target toxicities to non-hematopoietic tissues, which limit their broader application. Human CD34+ hematopoietic stem cells (HSCs) express high levels of CD45, which is restricted to the hematopoietic lineage. We previously demonstrated that anti-CD45 antibodies facilitated engraftment in children with immunodeficiency with minimal non-hematopoietic toxicity (Straathof et al Lancet 2009), but myeloid engraftment was suboptimal suggesting more potent killing of human HSCs is needed to extend this approach to immunocompetent patients. Additionally, since almost all hematological malignancies express CD45, targeting CD45 could potentially be used as an adjunctive cytoreductive bridge to SCT in patients with refractory leukemia. We have investigated the potential of anti-CD45 antibody-drug conjugates (ADCs) to target human HSCs and leukemia.
Methods. We generated anti-human CD45 ADCs from antibody YTH24.5 conjugated to SG3249 and SG3376 which share a highly potent pyrrolobenzodiazepine (PBD) dimer toxin with cleavable and non-cleavable linkers, respectively. Isotype control ADCs were also prepared. We assessed their in vitro cytotoxicity in 5-day cell viability assays on CD45+ and CD45- cell lines and in clonogenic assays where human CD34+ cells were incubated with varying concentrations of ADCs prior to plating in methylcellulose.
To assess depletion of human HSCs in vivo, NSG mice were humanized with 0.5x106 CD34+ cells from a healthy donor. Following engraftment, mice were conditioned with 1 mg/kg anti-CD45 ADC or controls. One week after treatment, human cells in the bone marrow, blood and spleen were analyzed by flow cytometry.
In tumor models, NSG mice were transplanted with an AML cell line (OCIM1) expressing firefly luciferase. A single dose of 1 mg/kg anti-CD45 ADC or controls were given iv before/after established tumor was detected. Tumor progression was followed by bioluminescence imaging.
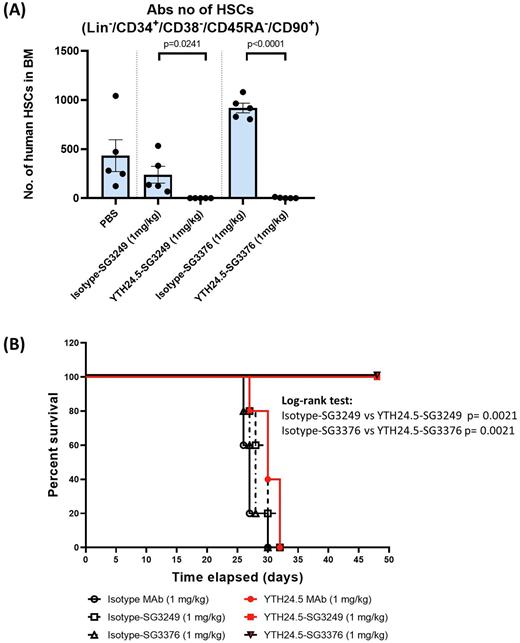
Results. Anti-CD45 ADCs YTH24.5-SG3249 and YTH24.5-SG3376 potently and specifically killed human CD45-expressing leukemic cell lines in cell viability assays, with EC50 values of 0.213 pM and 0.052 pM, respectively, and showed a wide (> 4 log) therapeutic window compared to CD45- cell lines. When a mixture of both CD45+ and CD45- cell lines was treated in vitro, the ADC with the non-cleavable linker (YTH24.5-SG3376) induced less bystander killing of CD45- targets. In clonogenic assays, both ADCs specifically inhibited colony formation of human CD34+ progenitors with EC50 values of 5-10 pM, compared to >17 nM for the isotype control ADCs. YTH24.5 ADCs had short half-life (36h) in NSG mice, which is critical if they are to be cleared before infusion of allogeneic/gene corrected HSCs. Importantly, a single dose of 1 mg/kg of either anti-CD45 ADC completely eradicated human HSCs in the bone marrow of humanized mice (p<0.05 for YTH24.5-SG3249 and p<0.0001 for YTH24.5-SG3376 vs Isotype ADCs, respectively) with no non-specific killing by the Isotype-SG3376 control (Fig. 1A). Moreover, the anti-CD45 ADCs also showed potent anti-leukemic activity and prevented the development of AML and prolonged survival (p<0.01 for both YTH24.5 ADCs vs Isotype ADC controls) in a xenogeneic OCIM1 model of AML in NSG mice (Fig. 1B). Further, in an established AML tumor model, anti-CD45 ADCs also induced tumor regression and prolonged survival (p<0.01 for YTH24.5-SG3249 and p<0.001 for YTH24.5-SG3376 vs Isotype ADC controls). Similar results were observed with a xenogeneic Jurkat model of T-ALL. Additional studies in a PDX model of AML are ongoing.
Conclusion. Our pre-clinical data show that CD45-specific ADCs with a PBD dimer toxin are highly effective at ablating human HSCs and show promise as targeted conditioning agents for gene therapy and allogeneic SCT, potentially avoiding the non-hematopoietic toxicity of conventional conditioning. Additionally, they have potent anti-leukemic activity suggesting they may also have a role in enabling transplantation in refractory hematological malignancies.
Fig. 1. A) Complete eradication of human HSCs in the bone marrow of humanized NSG mice treated with the anti-CD45 ADCs. Unpaired t-test p-values indicated. B) OCIM1 bearing mice treated with anti-human CD45 ADCs show prolonged survival compared to Isotype ADC or naked antibody.
Disclosures
Yeung:Quell Therapeutics: Consultancy; ADC Therapeutics: Patents & Royalties: WO2022063853A1 Named inventor; UCL Business: Patents & Royalties: WO2022064191A1 Named inventor; Bristows LLP, 100 Victoria Embankment, London EC4Y 0DH: Consultancy. Kirby:ADC Therapeutics: Current Employment, Current equity holder in private company. Zammarchi:ADC Therapeutics SA: Current Employment, Current equity holder in publicly-traded company. Havenith:Genmab: Patents & Royalties: Patent with Genmab; ADC Therapeutics: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. van Berkel:ADC Therapeutics: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Booth:Orchard Therapeutics: Consultancy; Revolution Medicines: Research Funding; Sobi: Honoraria. Gaspar:Orchard Therapeutics: Current Employment. Thrasher:Orchard Therapeutics: Consultancy; Rocket Pharmaceuticals: Consultancy; 4bio capital: Consultancy; Generation Bio: Consultancy, Current equity holder in publicly-traded company. Chester:Allen & Overy LLP: Consultancy; ADC Therapeutics: Patents & Royalties; Informa PLC: Membership on an entity's Board of Directors or advisory committees; UCL Business: Patents & Royalties; Novalgen: Consultancy, Current Employment, Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months, Patents & Royalties. Amrolia:Bluebird Bio: Research Funding; Pierre Fabre: Consultancy; UCL Business: Patents & Royalties; Bluebird Bio: Research Funding; Pierre Fabre: Consultancy; Autolus: Patents & Royalties, Research Funding; ADC Therapeutics: Patents & Royalties: named inventor WO2022063853A1.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal